Research - Batteries

BatteriesLi-S batteries will play an important role in enabling net zero energy transition by 2050. Li-S batteries offer five times more energy density than Li-ion batteries because the Li-S chemistry releases 16 electrons during battery operation. It is therefore essential to understand the lithium sulfur chemistry so that the performance of Li-S batteries can be translated into viable technology. Our research aims to accelerate the development of energy storage materials and devices based on metal-free, high-energy, fast-charging and long-life Li-S chemistry using metallic two-dimensional (2D) 1T phase MoS2. We are part of the LiSTAR (Lithium-Sulfur Technology Accelerator) project funded by the Faraday Institution. Our research contributes to:
Our breakthrough in Li-S pouch cell batteries has resulted in a successful spin-out company, Molyon, supported by the ERC Proof of Concept Grant and funding bodies. |
Ongoing Projects |
1. Li-S BatteriesOur recent work on Li-S battery cathodes have demonstrated that metallic 2D MoS2 is an ideal sulfur host material for high-performance Li-S batteries (Nature Energy, 2023). This material was found to be environmentally and thermally stable (Chemistry of Materials, 2024), which enables scaled production of practical Li-S pouch cell batteries. In addition, our observation of a quasi-solid-state interface which forms in-situ on the metallic 1T MoS2 host extends the lifetime of Li–S batteries beyond the state-of-the-art (ACS Nano, 2024).
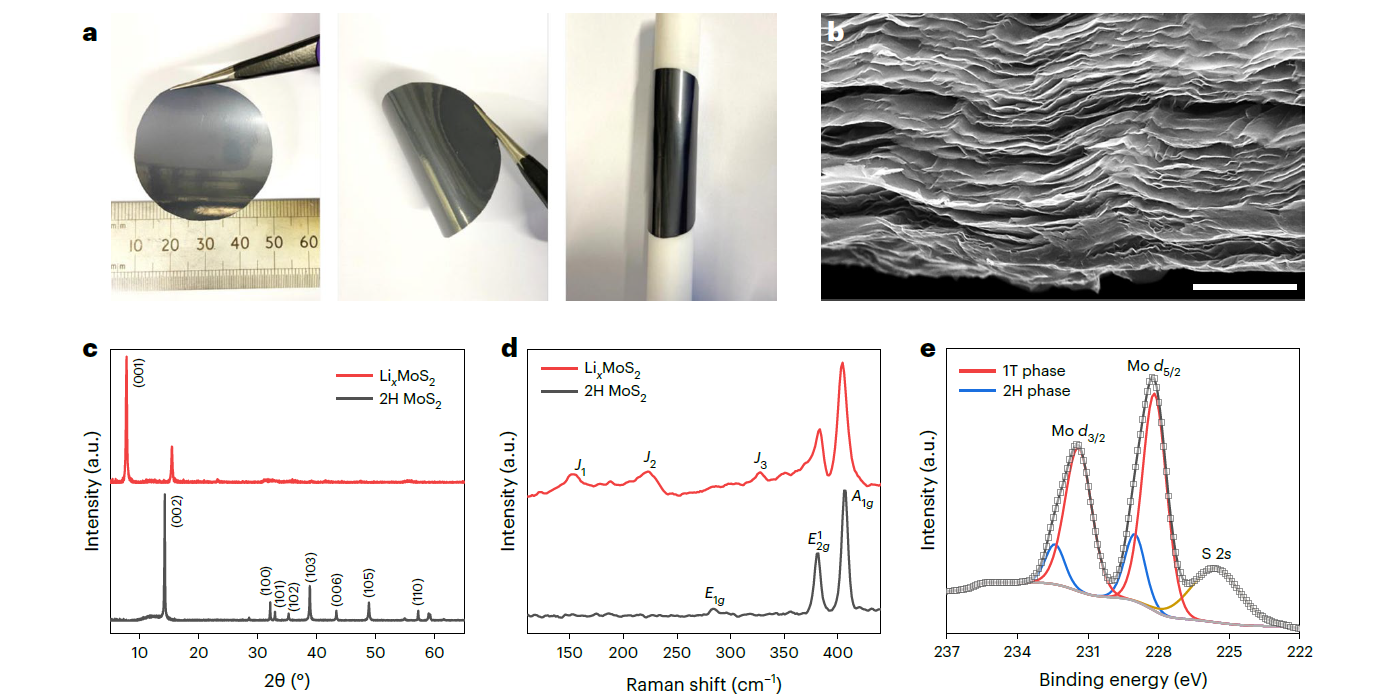
Figure 1. a) Photographs of a freestanding LixMoS2. b) Cross-sectional SEM image of the LixMoS2 film. c-e) In-depth material characterization of 2H MoS2 and LixMoS2 (Nature Energy 2023, 8, 84–93). |
2. Na-S BatteriesThe chemical reaction between sodium and sulfur gives Na-S batteries high energy densities at a reduced materials cost. We use Raman and UV-vis spectroscopies to observe formation of polysulfides during charge and discharge to determine the voltages at which these conversion reactions occur. Further research will involve developing a novel sulfur host specifically for Na-S batteries.
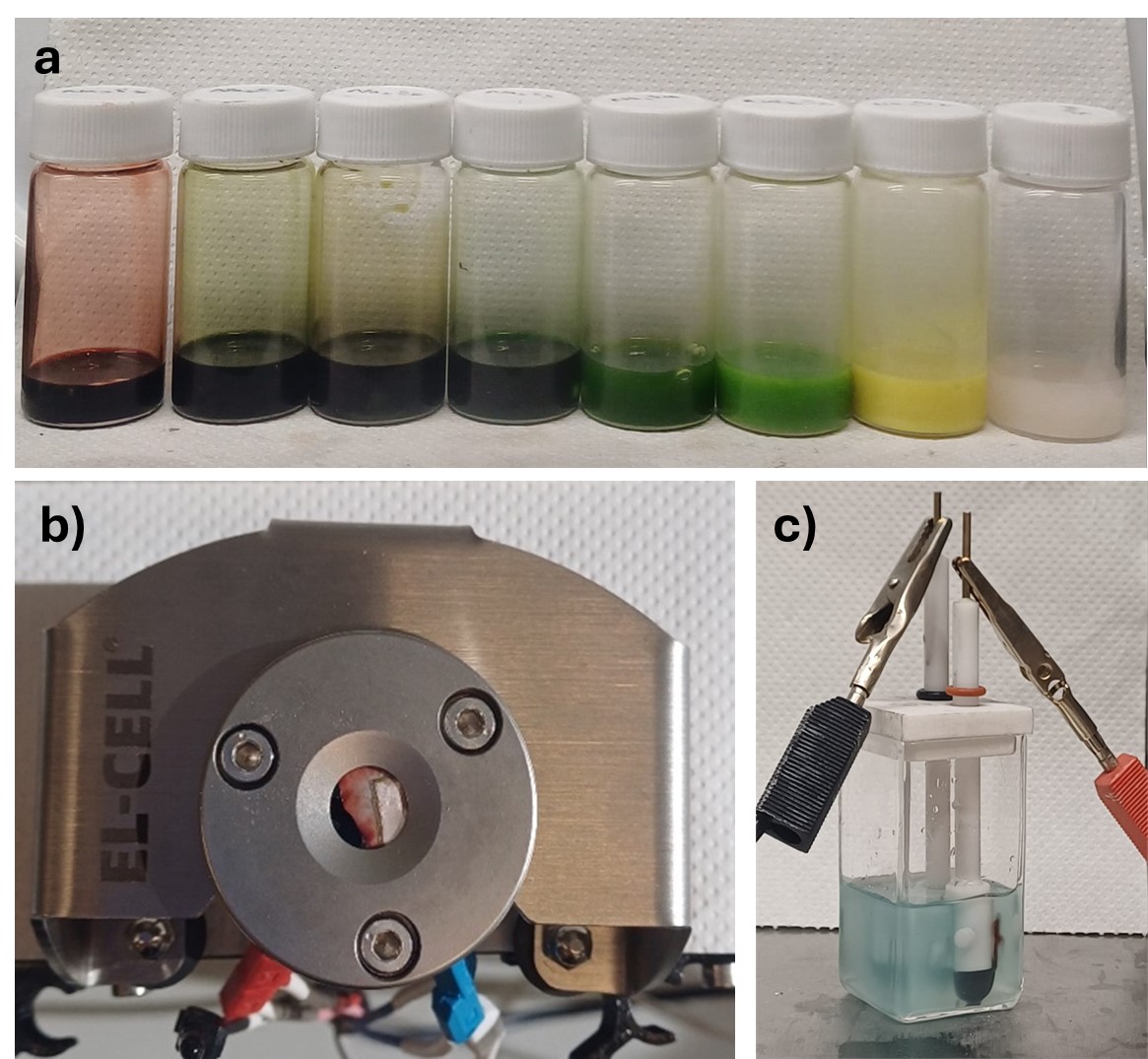
Figure 2. a) Solutions of standard sodium polysulfides prepared in electrolyte. b) Electrochemical set-up of in-situ Raman. c) Electrochemical set-up of ex-situ UV-vis. |
3. Li-Metal AnodeLi-metal based batteries are an attractive alternative to Li-ion batteries for their superior energy density. However, the problem associated with lithium metal anodes is the formation of Li dendrites, resulting in “dead” Li accumulation, poor coulombic efficiency, short cycle life, and hazardous safety risks. Our research focuses on studying the effect of surface modification on the lithium plating/stripping behaviour in lithium metal anode.
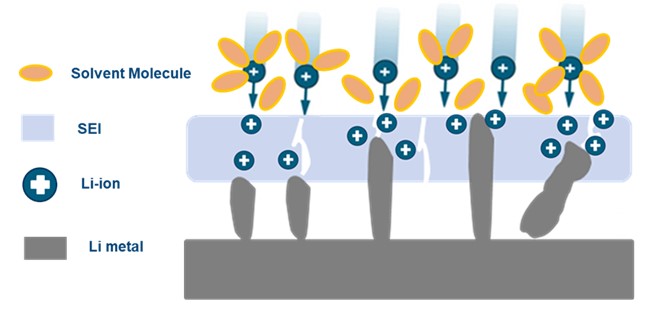
Figure 3. Schematic of Li deposition process. |
4. Solid-State ElectrolytesA current concern with state-of-the-art commercial batteries is the flammable nature of the liquid electrolyte. We are working on several solid-state alternatives that will improve the safety of batteries. Our group investigates polymer electrolytes such as PEO, ceramic electrolytes such as LLZO and nanoscale composites of these. The batteries we make now outcompete commercial batteries at high temperatures and we are currently focused on reducing the minimum operating temperature to below room temperature.
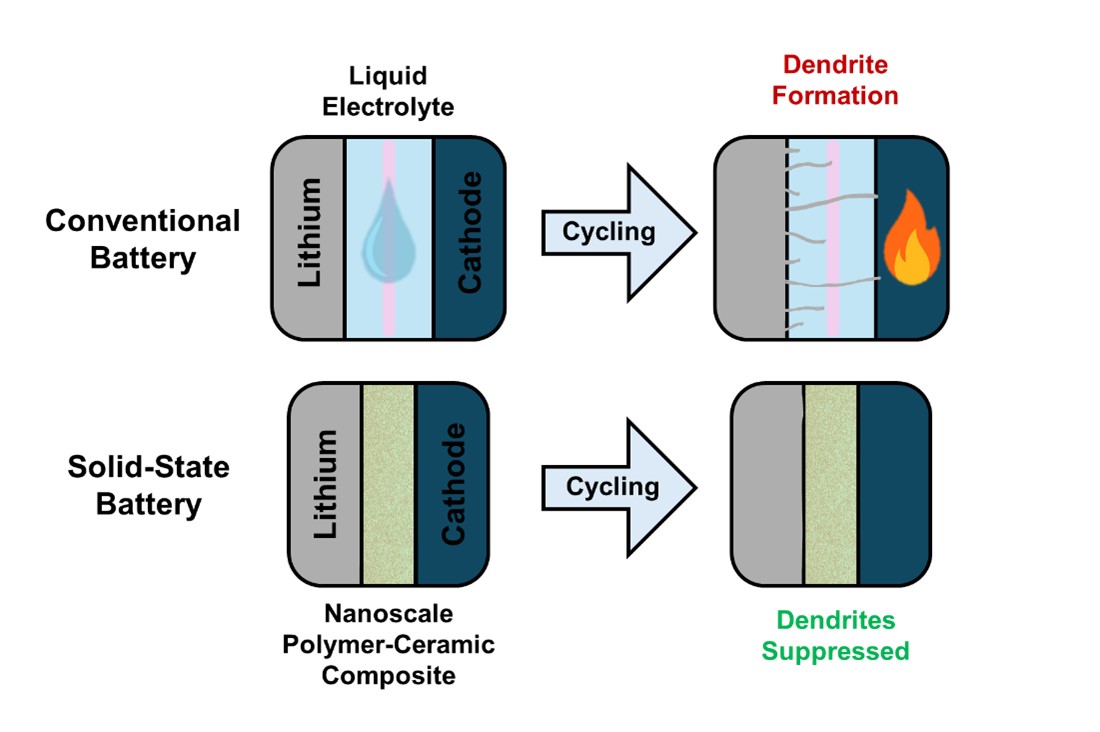
Figure 4. Lithium metal batteries are generally dangerous with liquid electrolytes because they pose no barrier to dendrites during growth. On repeated charge-discharge cycling, this can short the battery and may cause ignition of the electrolyte. Solid electrolytes form a physical barrier to suppress dendrites and are made of non-flammable materials. This enables the safe use of lithium metal anodes; therefore increasing the energy density. |
Articles from the Group |
1. Why are Solid-State Electrolytes Gaining Popularity?Many people consider all-solid-state batteries (batteries with a solid-state electrolyte rather than a conventional liquid electrolyte) to be the next step in battery technology and the funding in solid-state electrolyte research reflects this. By 2031, the market for solid-state electrolytes is expected to reach $8 billion with large companies such as Audi and BMW currently investing heavily. At first glance, this seems strange because solid-state electrolytes have a lower conductivity for Li-ions and are far more expensive and difficult to make - so is it all hype or is there something more going on?
Although the technology is not there yet, solid-state electrolytes promise to increase the energy density, power density and cycle lifetime of batteries whilst also making them more safe. In current, liquid electrolyte Li-ion batteries, every time the battery is charged and discharged, lithium is transferred from one electrode to the other. If a pure lithium anode is used, the lithium deposition becomes uneven leading to the formation of whisker-like lithium dendrites through the electrolyte. When these reach the cathode, the battery is shorted and no longer usable. Instead of pure lithium, lithium-containing carbon is used as an anode in commercial cells. This prevents lithium dendrites forming but means a lot of the anode is carbon and not lithium - the active material in the battery. Even more problematic is the risk of the liquid electrolyte catching fire or exploding. This occurs when the heat that is inevitably released during battery operation, heats the electrolyte to above its combustion temperature and the electrolyte ignites.
Solid electrolytes solve these problems because solids typically have a much higher combustion temperature and so are far less likely to ignite. They also form a physical barrier that may prevent lithium dendrites from growing through them. This means all-solid-state batteries are much safer and can use pure lithium anodes, achieving huge performance improvements. With approximately 500 kg of Li-ion batteries in a standard electric car, these improvements in safety and performance are critical for the successful growth of the electric vehicle industry and constitute the main reasons why car manufacturers are investing so heavily in the technology. This investment is primarily focussed on finding novel solid electrolyte materials with ionic conductivities to rival liquid electrolytes and integrating these materials into functional batteries. James Moloney, 14/9/2021 |