Research - Electrocatalysis

Ongoing Projects |
1. Water Splitting of 2D TMDs and TMOCsTMDs have emerged as promising non-precious metal catalysts for hydrogen evolution reaction (HER). Numerous studies have focused on the optimization and construction of catalysts. However, such methods are usually restricted by complex structure engineering. We aim to improve the catalytic performance of TMDs via built-in electric fields, providing a new perspective for catalyst design in future.
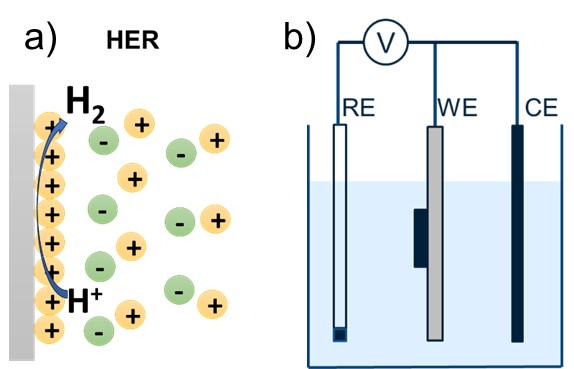
Figure 1. a) Schematic of hydrogen evolution reaction, and b) three-electrode setup.
In addition to TMDs, we have recently developed a method to synthesize transition metal oxychalcogenides (TMOCs) that are stable in acids. We have found that certain TMOCs are active for the oxygen evolution reaction at pH=0. TMOCs therefore hold promise for OER catalysts that are not based on expensive metals such as iridium.
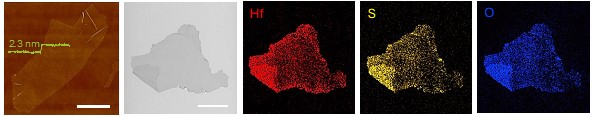
Figure 2. Microscopic imaging and chemical analysis of Hf-based TMOC. |
2. Spin-Dependent OERSpin-dependent electron transfer has been widely studied for OER catalysts. To delve into spin-dependent electron transfer mechanism within OER, we utilise two-dimensional (2D) vertical van der Waals heterostructures as catalysts for OER. Specifically, we employ 2D Fe3GeTe2 (FGT) as the catalyst and a thin layer of either graphene or hexagonal boron nitride (hBN) on top as the a non-magnetic spacer to separate the catalysts from the reactants.
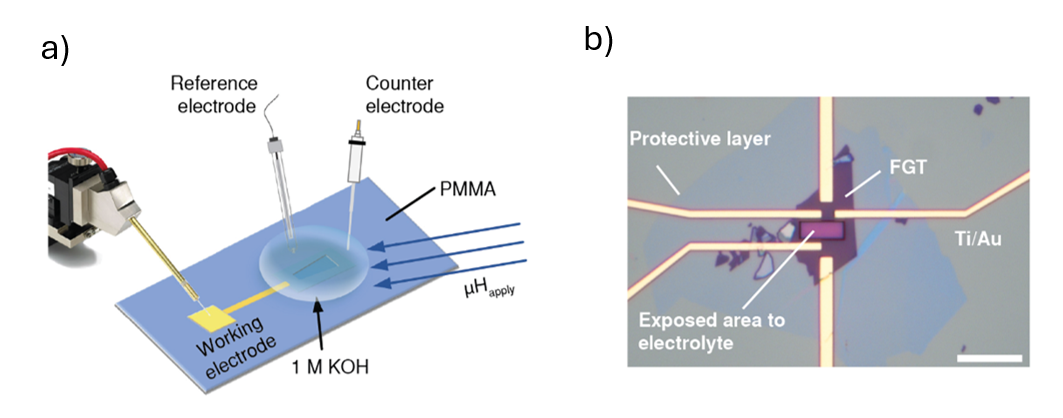
Figure 3. a) Schematic of micro-electrochemical cells for measuring OER in the presence of magnetic field. b) Optical microscope image of a heterostructure device. |