Research areas
Devices
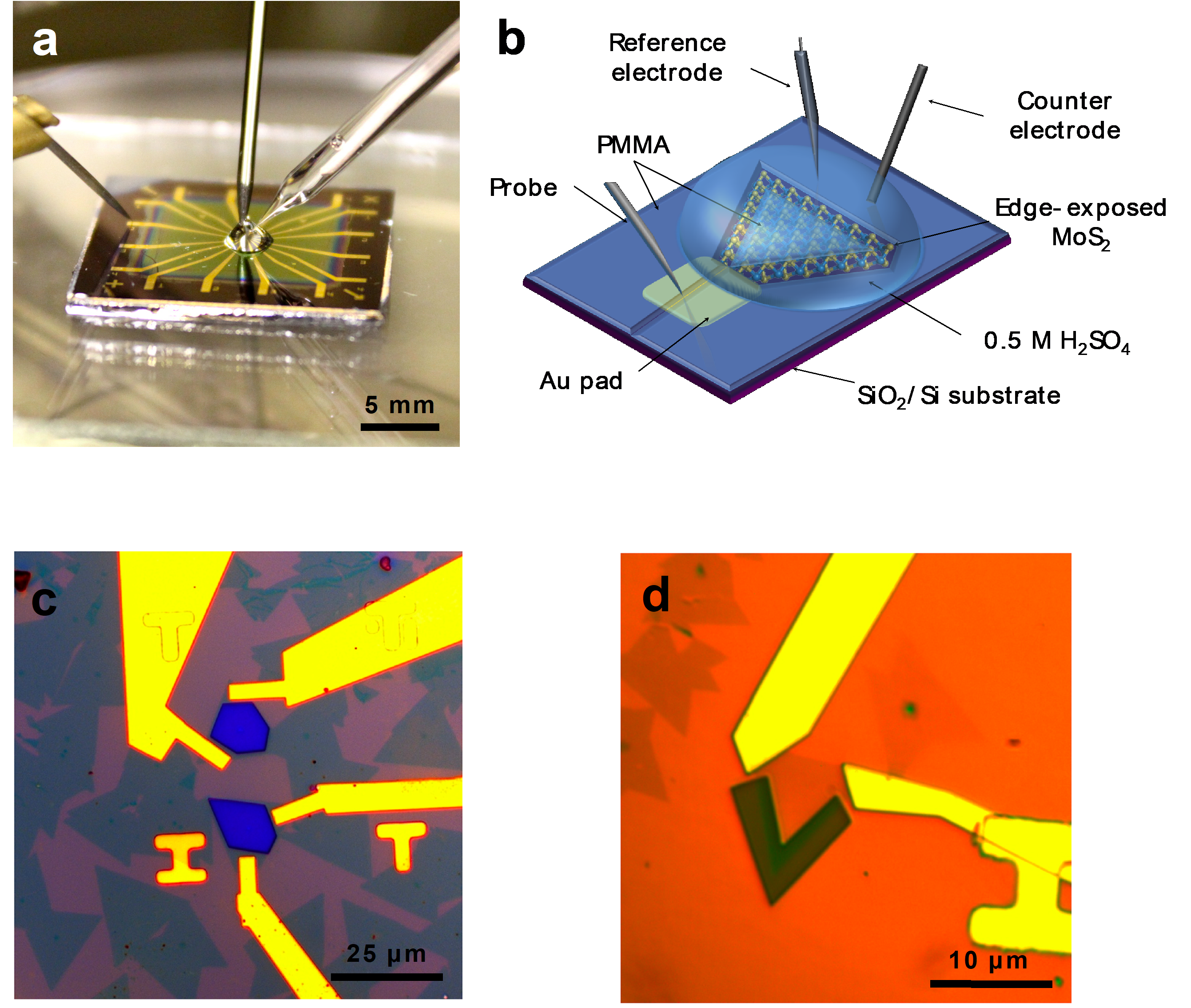
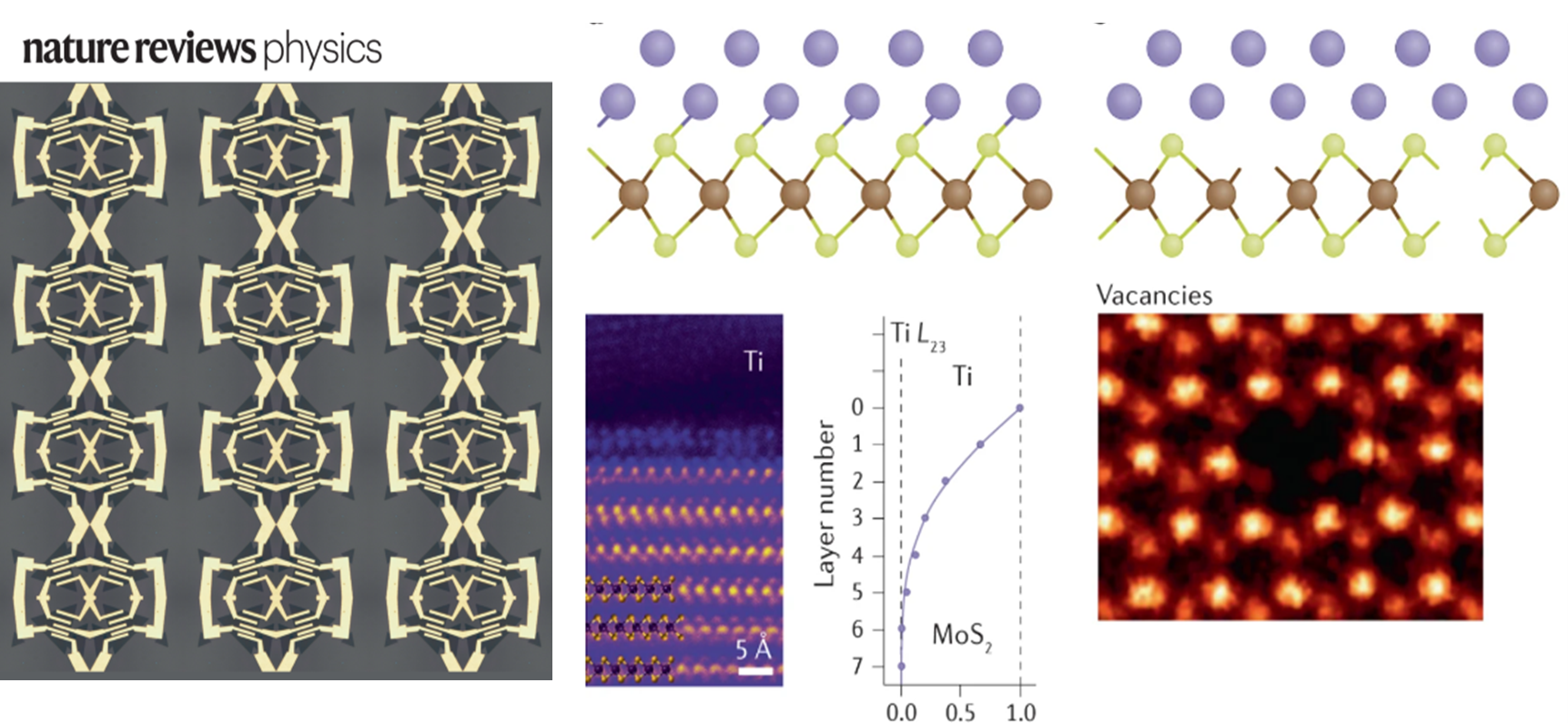
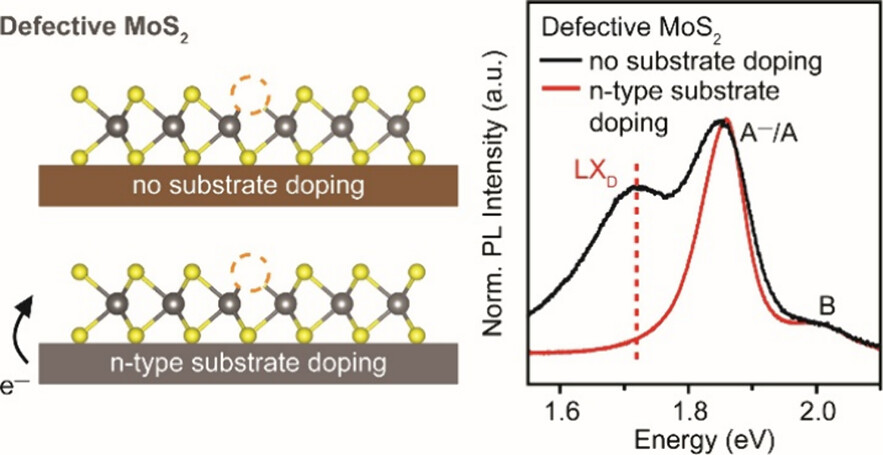
Atomically thin semiconductors: An emerging platform for ultra-low power electronics
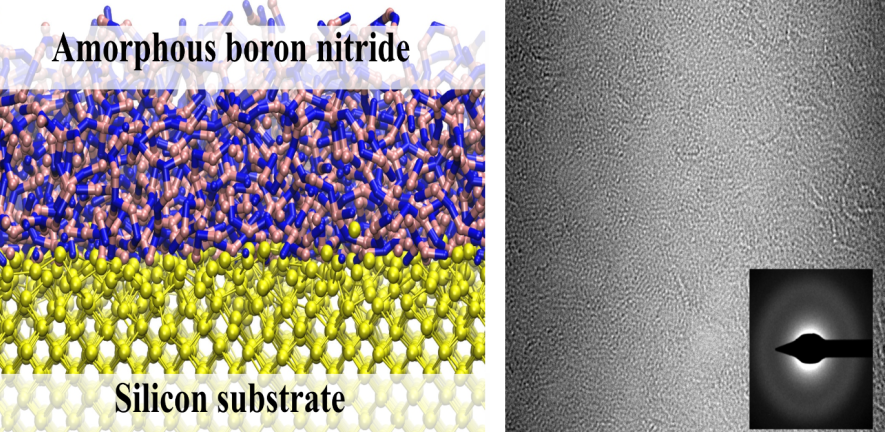
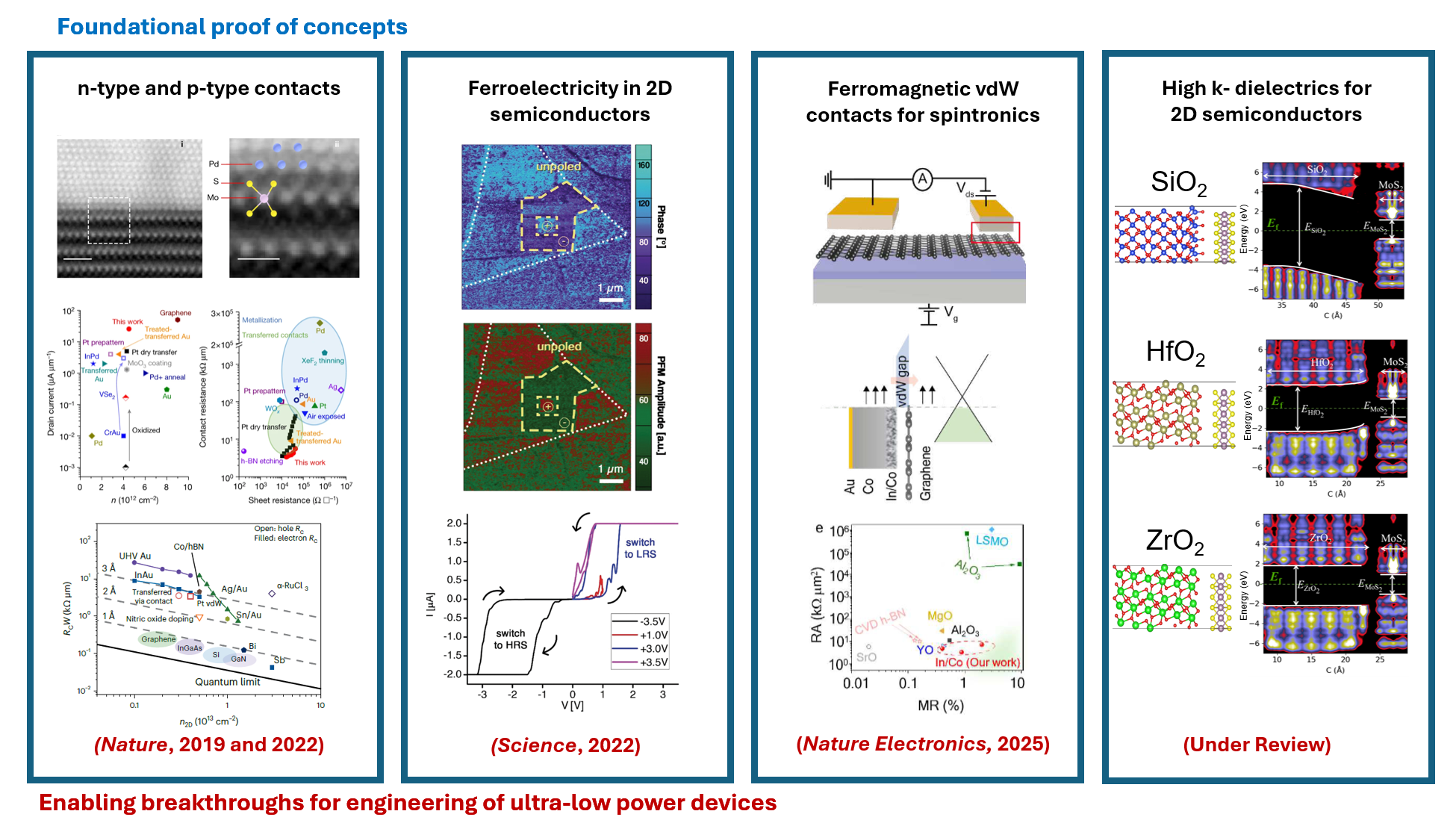
The carbon footprint (~4% of total CO2 emission) of modern electronics is comparable to that of aviation and is expected to rapidly increase due to the rise of artificial intelligence and data-centric approaches such as deep learning that are expected to consume a vast amount of energy. The proposed research aims to engineer the next generation of ultra-low power and energy efficient electronics. The programme is underpinned by our group’s recent breakthroughs in ultra-clean electrical contacts (Nature, 2019 & 2022) on atomically thin semiconductors, realisation of ultra-thin ferroelectric semiconductors (Science, 2022), and demonstration of large area single crystal hexagonal boron nitride as a universal dielectric layer for next generation of electronics (Nature, 2022), which provide the fundamental launching pad for technological innovation. These discoveries allow the realisation of new energy efficient electronic devices that consume very little power and are in principle compatible with the existing electronics infrastructure.
Batteries
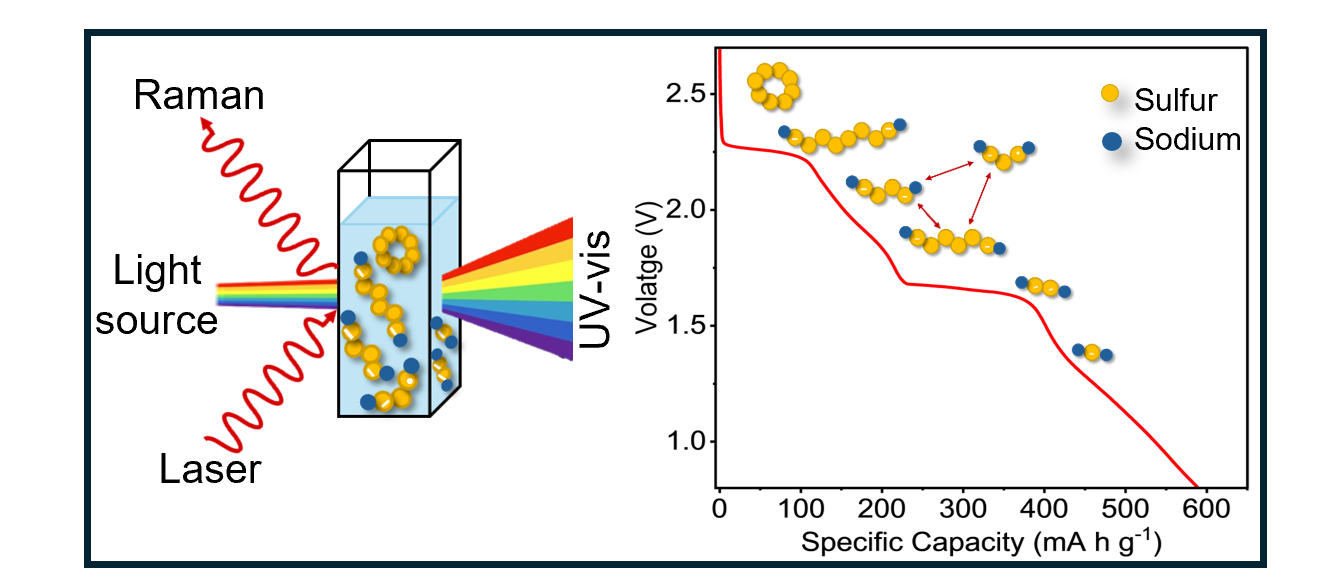
Li-S batteries will play an important role in enabling net zero energy transition by 2050. Li-S batteries offer five times more energy density than Li-ion batteries because the Li-S chemistry releases 16 electrons during battery operation. It is therefore essential to understand the lithium sulphur chemistry so that the performance of Li-S batteries can translated into viable technology. Our research aims to accelerate the development of energy storage materials and devices based on metal-free, high-energy, fast-charging and long-life Li-S chemistry. We are part of the LiSTAR (Lithium-Sulfur Technology Accelerator) project funded by the Faraday Institution.
Our research contributes to:
- Li-S and Na-S battery systems
- In-situ battery material characterization
- Li metal anode
- Solid-state electrolytes
- Na-ion batteries
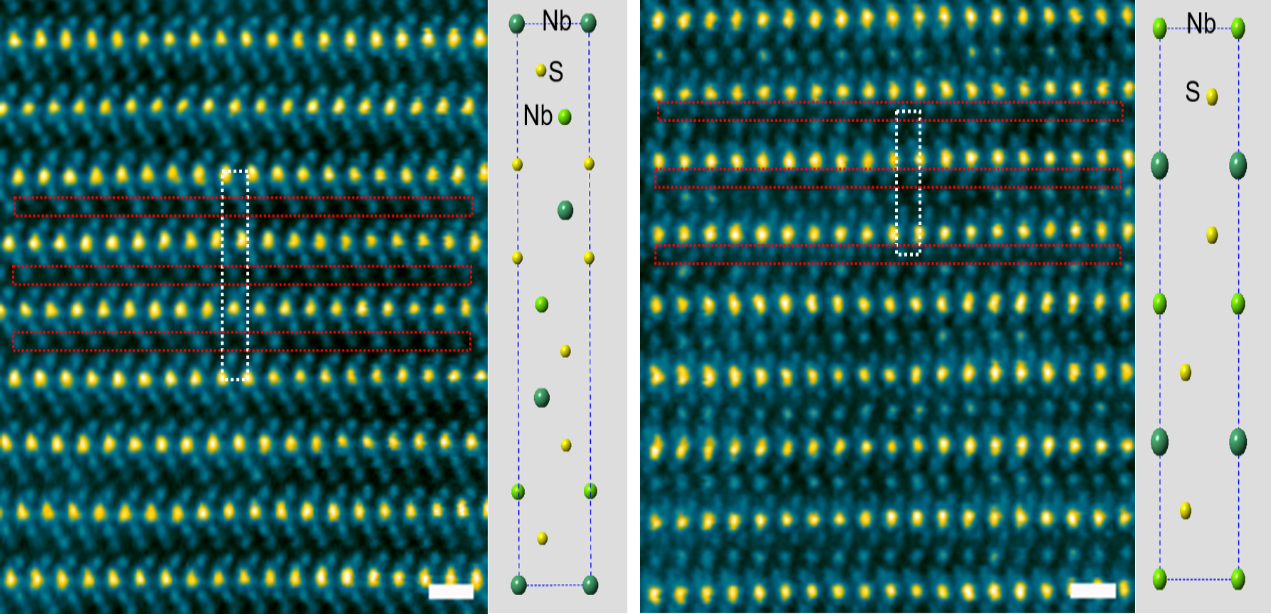
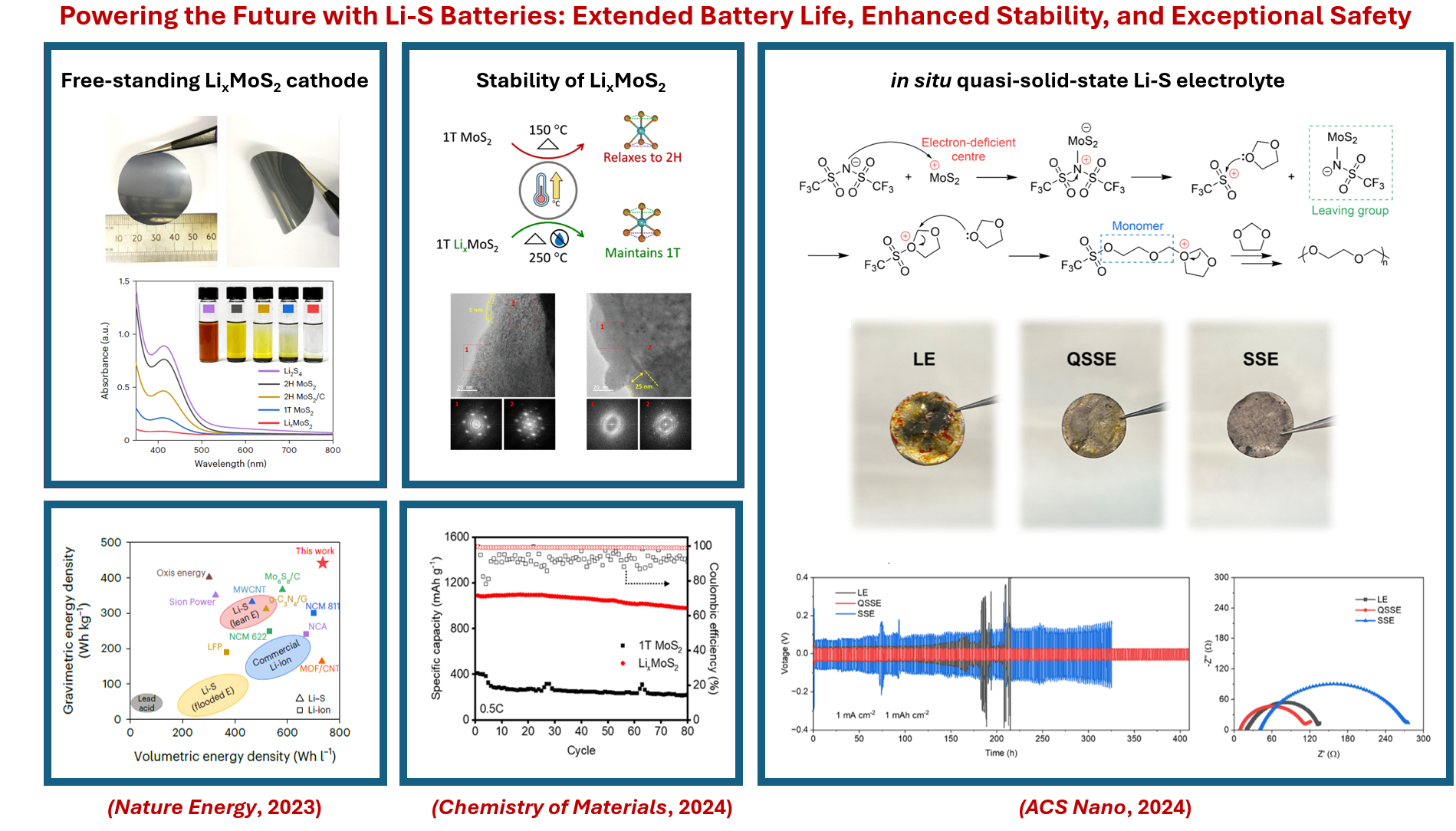
Our recent research includes developing a novel sulfur host material—lithiated 2D metallic MoS2 nanosheets—that enables high-performance Li–S batteries through its enhanced conductivity, electrocatalytic activity and lithophilic nature (Nature Energy, 2023); studying the environmental and thermal stability of LixMoS2 (Chemistry of Materials, 2024); and the in situ formation of a QSSE on the metallic 1T MoS2 host that extends the lifetime of Li–S batteries (ACS Nano, 2024).
Our breakthrough in pouch cell Li-S batteries has resulted in a successful spin-out company, Molyon, supported by the ERC Proof of Concept Grant and other funding bodies.
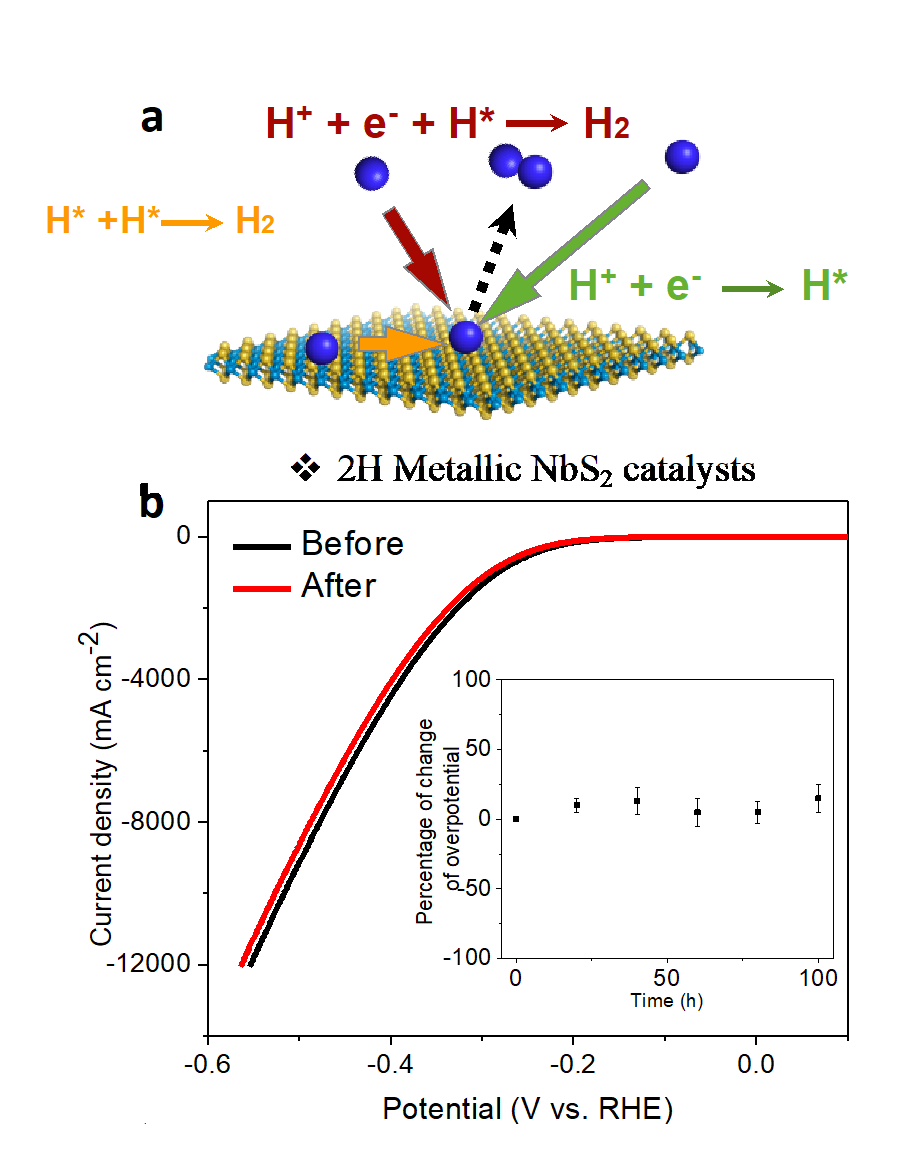
Electrocatalysis
Engineered 2D Transition Metal Dichalcogenides (TMDs) are highly active and affordable electrocatalysts that could significantly contribute to the widespread implementation of green hydrogen and other clean technologies. For instance, redox flow batteries, electrolyzers and fuel cells. Our material discovery methodology relies on finely tuning the structure, composition, and dimensions of different TMDs and composite materials as a viable tool to modulate substrate/catalyst interaction and associated catalytic activity. Among different reactions, we pay emphasis to new materials and testing methods to accelerate reactions including hydrogen evolution and oxidation, oxygen evolution and reduction, CO2 reduction or the electrosynthesis of ammonia via nitrogen activation.